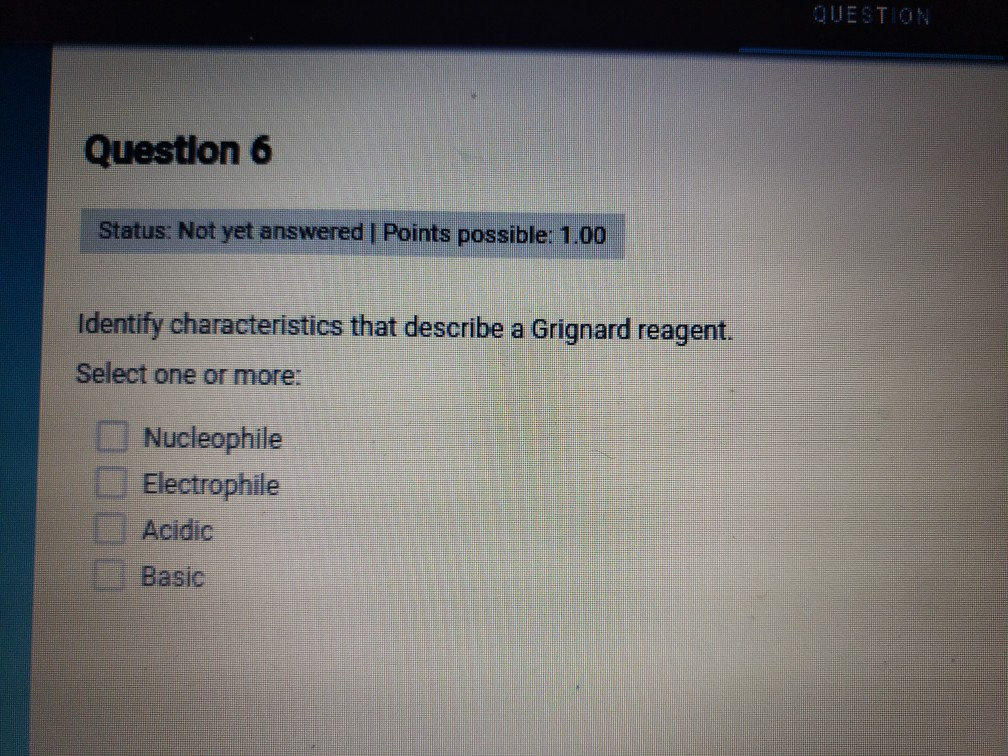
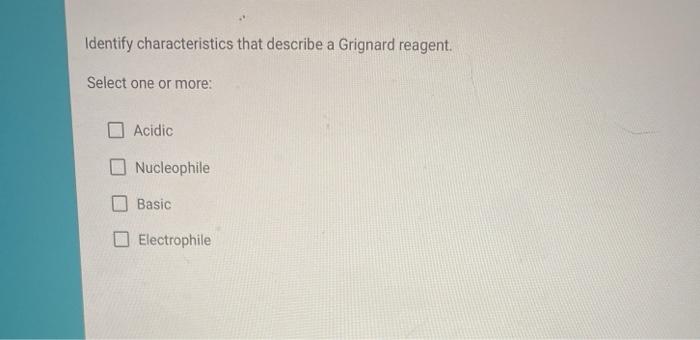
Grignard reagent definition any of the group of reagents produced by the interaction of magnesium and an organic halide usually in the presence of an ether and having the general formula RMgX where R is an organic group and X is a halogen. Grignard reagents are strong nucleophiles and can form carbon-carbon bonds making them somewhat similar to organolithium reagents.
Solved Question 4 Status Not Yet Answered Points Chegg Com
The reaction with formaldehyde leads to a primary alcohol.

. For the purposes of this page we shall take R to be an alkyl group. Grignard reagents are potent nucleophiles and react with electrophilic esters. They are called Grignard reagents after their discoverer French chemist Victor Grignard who was a corecipient of the.
A Grignard reagent or Grignard compound is a chemical compound with the generic formula RMgX where X is a halogen and R is an organic group normally an alkyl or aryl. Grignard reagent any of numerous organic derivatives of magnesium Mg commonly represented by the general formula RMgX in which R is a hydrocarbon radical. A grignard reagent is an extremely strong nucleophile and can behave like carbonyl compounds with electrophiles.
What is Grignard Reagent. Two typical examples are methylmagnesium chloride ClMgCH3 and phenylmagnesium bromide C6H5MgBr. It is important to.
Grignard is very unstable in the water and readily hydrolysis. Stir until all of the CO 2 has reacted and then allow the mixture to warm to room temperature. The addition of an excess of a Grignard reagent to an ester or lactone.
A Grignard reagent has a formula RMgX where X is a halogen and R is an alkyl or aryl based on a benzene ring group. And X is a halogen atom usually chlorine bromine or iodine. The anionic portion acts as a strong.
These are extremely important reagents developed by the French chemist Francois Auguste Victor Grignard who was awarded the Nobel Prize in 1912 in Chemistry for this work. 100 Identify characteristics that describe a Grignard reagent. For the purposes of this page we shall take R to be an alkyl group.
EXAMPLE Assume that in a preparation of triphenylmethanol you prepared phenylmagnesium bromide by reacting 21 mL of bromobenzene density 150 gmL with 050 g of magnesium in anhydrous ether. We review their content and use your feedback to keep the quality high. The organomagnesium compounds formed by the reaction of an alkyl or aryl halide with magnesium are called Grignard reagents.
The carbon of a carbonyl is electrophilic and even though the carbon is a secondary carbon atom because it is planar it is an excellent site for a nucleophilic attack. Grignard reagents are used synthetically to form new carboncarbon bonds. A Grignard reagent has a very polar carbonmagnesium bond in which the carbon atom has a partial negative charge and the metal a partial positive charge.
Grignard reagent is used to extend carbon chain in organic chemistry. The initial attack gives rise to a tetrahedral intermediate which collapses to give a ketone and bromomagnesium ethoxide. Grignard reagent reacts with many organic compounds and give different organic compounds.
Reaction of the Grignard reagent with CO 2 Transfer the Grignard reagent solution to a beaker. X Cl Br I. 100 3 ratings Transcribed image text.
Those positively charged hydrogen atoms are. Defining key concepts - ensure that you can accurately classify Grignard reagents. Fill a small glass sample vial with crushed solid CO 2 and add it slowly piece-wise to the Grignard reagent solution with rapid stirring.
You can titrate the Grignard reagent following the procedure of Watson and Eastham in J. The resulting ketone rapidly reacts with a second equivalent of Grignard reagent giving rise to the tertiary alkoxide. They are a subclass of the organomagnesium compounds.
Grignard Reagents are also used in the following important reactions. Not yet answered Points possible. The organomagnesium halides are known as Grignard reagents.
Use the provided 1H-NMR spectrum to determine the byproduct of this Grignard reaction. A Grignard reagent can be considered as an ionic compound with a magnesium halide cation and an organic anion. This quiz and worksheet allow students to assess the following.
A Grignard reagent has a formula RMgX where X is a halogen and R is an alkyl or aryl based on a benzene ring group. When an amido group substituent is used instead of the alkyl substituent amido magnesium halides are. The Grignard reagent is represented as R-Mg-X where.
Grignard reagent preparing reactions physical properties. R alkyl aryl alkenyl allyl group. The Grignard Reaction is the addition of an organomagnesium halide Grignard reagent to a ketone or aldehyde to form a tertiary or secondary alcohol respectively.
Alcohol we will react our Grignard reagent with a ketone more specifically benzophenone. The limiting reagent in a Grignard reaction is usually the substance to which you add the Grignard reagent but you have to confirm this by calculation. A grignard forms a violet.
A Grignard reagent has a formula RMgX where X is a halogen and R is an alkyl or aryl based on a benzene ring group. Hydrogen atom in water molecule is positively charged. Identify characteristics that describe a Grignard reagent.
The technique used to separate the pure product from any excess reagent impurities and byproducts NMR spectroscopy IR spectroscopy Melting point Chromatography Recrystallization QUESTION Question 6 Status. CH3 C2H5 C6H5 etc. Green identify the total number of atoms in conjugation.
A typical Grignard reagent might be CH 3 CH 2 MgBr. 2 pts g A common byproduct of Grignard reagent formation highlighting that the mechanism is more complicated than often depicted is a combination of two Grignard reagents. Griganard reagent R-MgX here R alkyl group X ClBrI is prepared by the reaction of alkyl halides halo alkanes and magnesium in dry ether medium.
Experts are tested by Chegg as specialists in their subject area. A typical Grignard reagent might be CH 3 CH 2 MgBr. For the purposes of this page we shall take R to be an alkyl group.
A typical Grignard reagent might be CH 3 CH 2 MgBr. Alkyl group of grignard reagent is a nucleophile and like to attack positive parts. As you will see throughout the remainder of this course Grignard reagents can be used to synthesize a wide range of organic compounds and are extremely useful to the organic chemist.
Solved Identify Characteristics That Describe A Grignard Chegg Com

Organic Chemistry Ii Lab 8 Grignard Reaction Synthesis Flashcards Quizlet

0 Comments